Petit Mal Treatment Market Size, Share, Trends & Growth Forecast 2032
Petit mal (absence seizures) is a type of generalized epilepsy characterized by brief episodes of staring, unresponsiveness, and subtle motor symptoms (eye blinking, lip smacking), most common in children aged 4–14 years. Treatment primarily involves anti-epileptic drugs (AEDs) such as ethosuximide (first-line), valproic acid, lamotrigine, levetiracetam, and topiramate, with adjunctive therapies, ketogenic diet, or vagus nerve stimulation in refractory cases. The global market grows steadily due to increasing epilepsy diagnosis rates, pediatric neurology awareness, orphan drug incentives for rare epilepsy syndromes, pipeline development of next-generation AEDs with better tolerability, and rising access to EEG diagnostics in emerging markets. North America dominates (largest share) from high diagnosis rates, FDA approvals, strong reimbursement for AEDs, and specialized pediatric epilepsy centers, while Asia-Pacific is the fastest-growing region due to large pediatric population, improving healthcare access, and rising epilepsy prevalence in India and China.
Market Size and Growth Projections
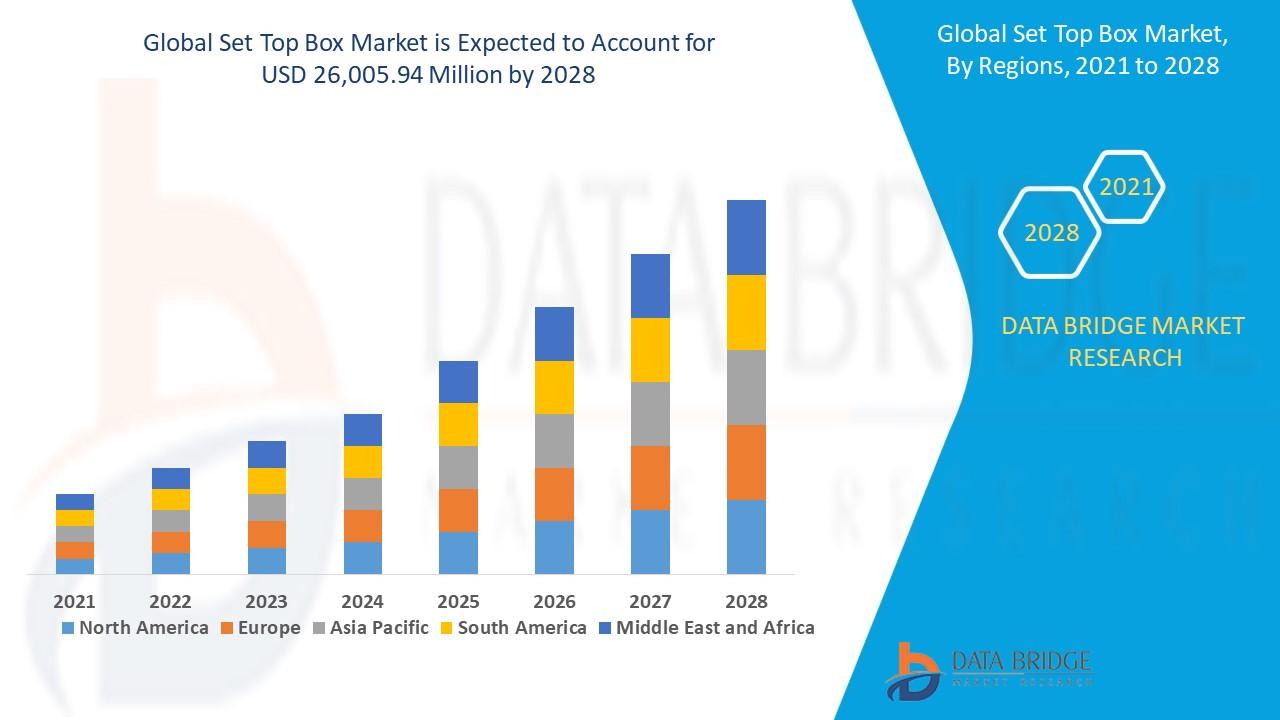
The global petit mal treatment market was valued at USD 456.78 million in 2024 and is projected to reach USD 812.34 million by 2032, growing at a compound annual growth rate (CAGR) of 7.4% during the forecast period from 2025 to 2032. This moderate growth reflects stable demand for first-line AEDs, pipeline innovation, and increasing diagnosis in developing regions.
Market Segmentation
The market is segmented as follows:
- By Drug Type: Ethosuximide (dominant share in 2025; first-line for typical absence seizures), Valproic Acid/Valproate, Lamotrigine, Levetiracetam, Topiramate, Zonisamide, Clobazam, Others (emerging pipeline agents).
- By Route of Administration: Oral (dominant; tablets, syrups for pediatric use), Injectable (used in acute settings), Others.
- By Age Group: Pediatric (4–14 years – largest share; peak incidence), Adolescent & Adult (fastest-growing; persistent or late-onset cases).
- By Distribution Channel: Hospital Pharmacies (dominant), Retail Pharmacies, Online Pharmacies (fastest-growing), Specialty Pharmacies.
- By End User: Hospitals & Specialty Clinics (largest share), Homecare, Neurology Centers, Others.
- By Region: North America (largest revenue share; U.S. high diagnosis & reimbursement), Asia-Pacific (fastest-growing; India/China pediatric population & access), Europe, Latin America, Middle East & Africa.
Key Drivers Fueling Growth
- Rising incidence and diagnosis of absence seizures in children due to better EEG availability.
- Established efficacy and first-line status of ethosuximide and valproate.
- Growing pipeline of second-generation AEDs with improved safety profiles.
- Increasing awareness among parents and pediatricians for early intervention.
- Government programs for epilepsy care and orphan drug incentives.
- Expansion of tele-neurology and remote monitoring in developed markets.
Challenges and Restraints
- Side effects of AEDs (cognitive impairment, weight gain, behavioral issues in children).
- Limited treatment options for refractory or atypical absence seizures.
- High cost of branded AEDs and limited reimbursement in emerging markets.
- Diagnostic challenges (absence seizures often misdiagnosed as inattention/ADHD).
- Regulatory hurdles for new AED approvals in pediatric populations.
Get Full Access Of The Report: https://www.databridgemarketresearch.com/reports/global-petit-mal-treatment-market
Opportunities
- Strong pipeline of novel AEDs and combination therapies for refractory cases.
- Growth in Asia-Pacific from increasing pediatric neurology access.
- Expansion of generic AEDs improving affordability in developing regions.
- Rising demand for extended-release formulations and better-tolerated drugs.
- Integration of digital tools for seizure tracking and medication adherence.
- Opportunities in rare epilepsy syndromes with absence features.
Competitive Landscape
The market is led by established neurology/aED manufacturers with strong pediatric portfolios and generic competition. Key players include:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Dr. Reddy’s Laboratories Ltd. (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Eisai Co., Ltd. (Japan)
- UCB S.A. (Belgium)
- Supernus Pharmaceuticals, Inc. (U.S.)
- Zogenix (now Jazz Pharmaceuticals) (U.S.)
- SK Life Science (U.S.)
- Aquestive Therapeutics (U.S.)
- Others (generic and regional AED suppliers)
Future Trends and Opportunities
Trends include next-generation AEDs with fewer cognitive side effects, extended-release formulations, digital seizure diaries/apps, and personalized medicine based on EEG/genetic biomarkers. Opportunities are strongest in Asia-Pacific access expansion, generic penetration, and refractory/absence seizure pipeline.
Conclusion
The global petit mal treatment market is positioned for reliable growth through 2032, driven by pediatric epilepsy awareness, established AEDs, and pipeline innovation—led by North America and fastest-growing in Asia-Pacific. While side effects and diagnostic challenges remain, opportunities in generics, emerging markets, and next-gen therapies offer strong potential. Stakeholders should prioritize pediatric safety, affordability, and digital adherence tools to address this childhood neurological need.
Browse More Reports:
Global Imitation Jewellery Market
Global Industrial Cybersecurity Market
Global AI Meeting Assistants Market
Global Predictive Maintenance Market
Global Underwater Robotics Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today! Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- corporatesales@databridgemarketresearch.com