Healthcare Regulatory Affairs Outsourcing MarketSize and Growth Forecast: Emerging Trends & Analysis
The Global Healthcare Regulatory Affairs Outsourcing Market is witnessing robust expansion as pharmaceutical, biotechnology, and medical device companies increasingly rely on external expertise to navigate complex regulatory landscapes. With escalating regulatory demands, growing R&D investments, and heightened pressure to accelerate product approvals, outsourcing regulatory services has become a strategic and cost-efficient solution.
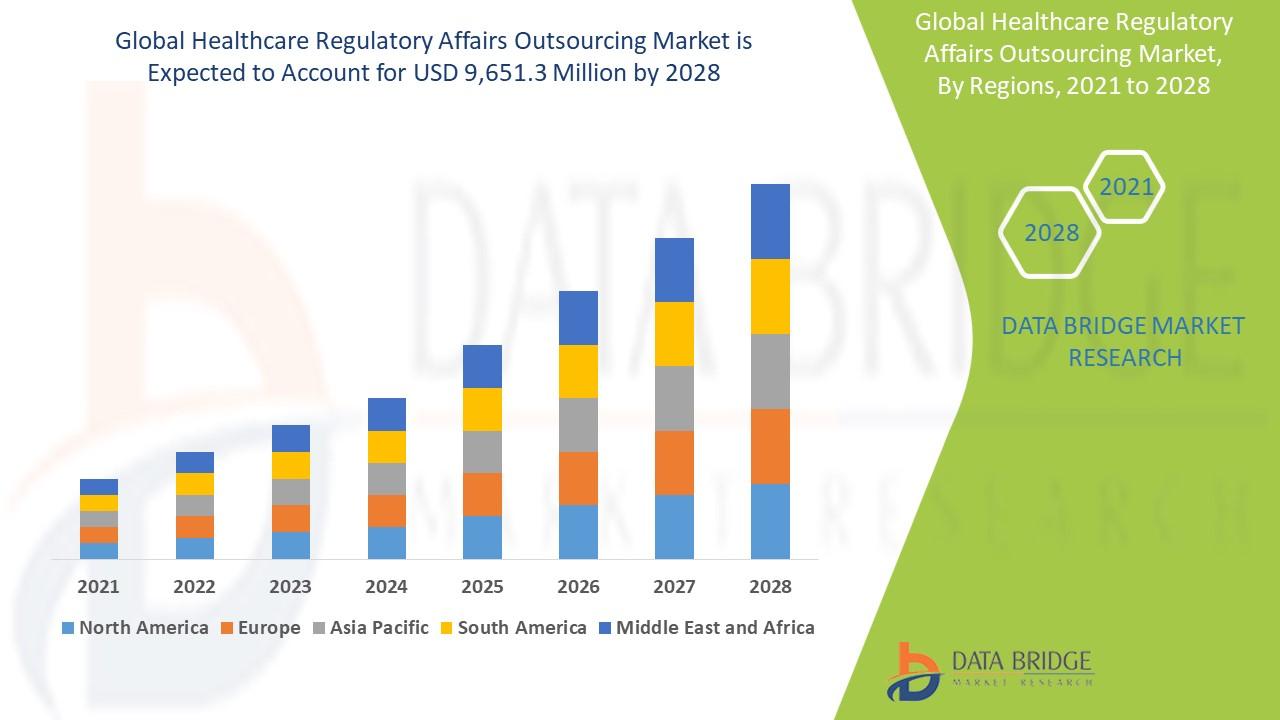
The global Healthcare Regulatory Affairs Outsourcing market size was valued at USD 6.42 billion in 2024 and is projected to reach USD 14.49 billion by 2032, with a CAGR of 10.71% during the forecast period of 2025 to 2032.
Market Overview
Regulatory affairs outsourcing includes services such as product registration, clinical trial application (CTA) support, regulatory documentation, legal representation, and post-marketing surveillance. Companies opt for outsourcing to ensure compliance with standards set by regulatory bodies such as FDA (U.S.), EMA (Europe), PMDA (Japan), WHO, and other global agencies, especially during drug development, approval, and commercialization.
The market is expected to grow significantly owing to increasing globalization of clinical trials, tightening regulatory frameworks, and the rising complexity of healthcare products such as biologics, combination therapies, and digital therapeutics.
Key Growth Drivers
Increasing complexity of regulatory requirements across multiple regions.
Rising R&D investments in pharmaceuticals and biotechnology.
Acceleration in drug and medical device approvals, creating demand for expert compliance support.
Growth in clinical trial activities across emerging economies.
Cost efficiency and scalability benefits of outsourcing over in-house regulatory teams.
Growing demand for regulatory consulting during mergers & acquisitions.
Adoption of digital transformation and regulatory intelligence solutions.
Market Segmentation
By Service Type
Regulatory Writing & Publishing
Product Registration & Market Authorization
Legal Representation
Regulatory Consulting
Life Cycle Management
Clinical Trial Applications & Submissions
Others
By End User
Pharmaceutical Companies
Biotechnology Firms
Medical Device Manufacturers
Contract Research Organizations (CROs)
Healthcare IT & Consulting Firms
By Region
North America – leads due to stringent FDA standards and the presence of major market players
Europe – high demand for EMA compliance and CE marking for devices
Asia-Pacific – fastest growing due to expanding clinical trials and cost advantage
Latin America & Middle East – rising regulatory standardization and healthcare modernization
Challenges
Variability in regulations across different countries
Data security and confidentiality concerns
Limited in-house regulatory knowledge in developing regions
Frequent policy changes and compliance updates
Opportunities
Increasing demand for AI-based regulatory intelligence tools
Expansion in orphan drug and rare disease regulatory services
Outsourcing for digital health products and telemedicine compliance
Growth in post-approval and life cycle management services
Recent Trends
Integration of Regulation-as-a-Service (RaaS) business models
Growing focus on BREXIT regulatory adjustments
Rising adoption of eCTD (electronic Common Technical Document) submissions
Strategic partnerships between CROs and regulatory consultants
Increased demand for regulatory compliance with companion diagnostics and personalized medicine
Competitive Landscape
Key players in the market include:
IQVIA Inc.
Parexel International Corporation
PharmaLex GmbH
WuXi AppTec
Icon PLC
Charles River Laboratories
ProPharma Group
Labcorp Drug Development
Companies are continuously expanding service portfolios and leveraging advanced data analytics and automation to improve decision-making and compliance efficiency.
Future Outlook
The Global Healthcare Regulatory Affairs Outsourcing Market is poised for substantial growth driven by:
✔ Increasing demand for faster product approvals
✔ Expansion of clinical research in emerging markets
✔ Rising adoption of regulatory digital transformation
✔ Growing complexity of healthcare products and compliance needs
Companies partnering with experienced regulatory outsourcing firms will gain a competitive edge by accelerating time-to-market while minimizing risks and regulatory delays.
Browse More Reports:
North America Physical Security Market
Middle East and Africa Offsite Sterilisation Service Market
Europe MRI scanner Market
Europe Medical Device Sterilization Market
North America Medical Device Sterilization Market
North America Injectable Drug Delivery Market
Middle East and Africa Hyaluronic Acid Market
Europe Hyaluronic Acid Market
U.S. Healthcare Analytics Market
Europe Healthcare Analytics Market
Middle East and Africa Espresso Coffee Market
Asia-Pacific Espresso Coffee Market
Asia-Pacific Electroencephalography Devices Market
Europe Burn Care Market
Europe Breast Biopsy Devices Market
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Conclusion
As the global healthcare landscape evolves, outsourcing regulatory affairs is becoming essential for managing compliance complexities, reducing operational costs, and supporting innovation. Strategic collaborations with regulatory experts and use of digital compliance tools will be key to sustainable growth in the coming years.