Europe Medical Device Sterilization MarketPotential: Size, Share, Trends, and Future Outlook
The Europe Medical Device Sterilization Market is experiencing steady growth due to expanding medical device production, rising surgical procedures, stricter regulatory requirements, and the increasing emphasis on infection prevention and control across healthcare facilities. Europe remains one of the world’s leading regions in medical device innovation and manufacturing, driving consistent demand for reliable, validated sterilization processes.
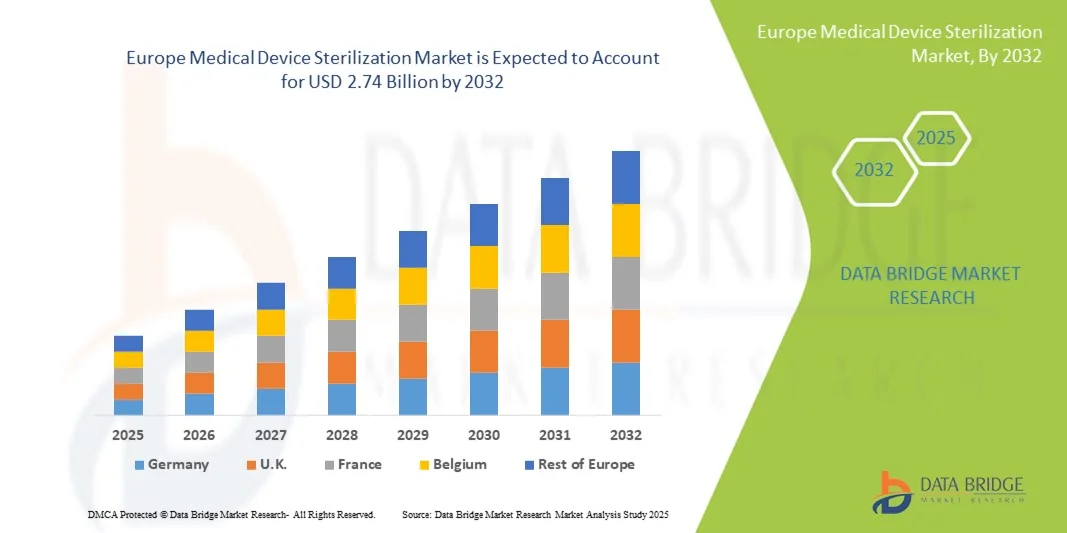
- The Europe medical device sterilization market size was valued at USD 1.47 billion in 2024 and is expected to reach USD 2.74 billion by 2032, at a CAGR of 8.10% during the forecast period
Market Overview
Sterilization is a critical process to ensure the safety, efficacy, and regulatory compliance of medical devices. In Europe, sterilization solutions are used extensively by:
-
Medical device manufacturers
-
Hospitals and clinics
-
Pharmaceutical companies
-
Laboratories and research centers
Regulatory bodies such as the European Medicines Agency (EMA) and European Commission (EC) enforce strict sterilization and contamination control standards, supporting market maturity and growth.
Key Market Drivers
1. Expansion of Medical Device Manufacturing
Europe, particularly Germany, Switzerland, the UK, and France, is a hub for medical device production. The rising need for sterile, safe, and high-quality devices—especially single-use products—is significantly boosting sterilization demand.
2. Increasing Surgical and Diagnostic Procedures
The region’s ageing population and high prevalence of chronic diseases have led to a surge in:
-
Orthopedic surgeries
-
Cardiovascular procedures
-
Diagnostic imaging interventions
-
Outpatient and minimally invasive surgeries
These procedures require sterilized instruments and devices, driving market growth.
3. Strict Regulatory Standards for Sterility Assurance
European regulations require:
-
Validated sterilization cycles
-
Documentation and traceability
-
Compliance with ISO 11135, ISO 17665, and ISO 11137 standards
This creates a strong demand for advanced sterilization technologies that ensure consistency and safety.
4. Rise in Hospital-Acquired Infections (HAIs) Prevention Programs
Hospitals in Europe increasingly prioritize sterilization to minimize the risk of infections, prompting investment in both in-house and outsourced sterilization services.
Market Segmentation
By Method
-
Ethylene Oxide (ETO) Sterilization
-
Steam Sterilization (Autoclave)
-
Radiation Sterilization
-
Gamma radiation
-
Electron beam (E-beam)
-
X-ray
-
-
Hydrogen Peroxide Plasma
-
Dry Heat Sterilization
By End User
-
Medical device manufacturers
-
Hospitals and clinics
-
Pharmaceuticals & biotechnology companies
-
Research and academic laboratories
By Service Type
-
In-house sterilization
-
Outsourced sterilization services
By Country
-
Germany
-
UK
-
France
-
Italy
-
Spain
-
Netherlands
-
Switzerland
-
Rest of Europe
Key Market Trends
1. Growing Adoption of Advanced and Low-Temperature Sterilization Technologies
With the increasing use of heat- and moisture-sensitive medical devices, demand for hydrogen peroxide plasma and ETO sterilization continues to rise.
2. Shift Toward Outsourcing Sterilization
Healthcare facilities and manufacturers are outsourcing sterilization to specialized third-party service providers to:
-
Reduce operational costs
-
Enhance documentation and validation
-
Ensure compliance with regulatory standards
3. Increasing popularity of Radiation Sterilization
Gamma and e-beam sterilization are gaining traction for large-scale medical device production due to:
-
Rapid processing time
-
Deep penetration
-
High reliability
4. Integration of Digital Monitoring and Analytics
Europe is witnessing greater adoption of:
-
RFID-enabled tracking
-
Automated sterilization workflows
-
Real-time process monitoring
-
Comprehensive cycle documentation
These improvements enhance traceability and quality assurance.
5. Sustainability and Environment-Friendly Solutions
European companies are investing in:
-
Reduced-EtO sterilization methods
-
Energy-efficient sterilization equipment
-
Improved waste and emissions management
Challenges
1. High Installation and Operational Costs
Advanced sterilization equipment and validation systems require significant investment.
2. Regulatory Complexity
Navigating multiple EU-wide and country-specific regulations can be challenging for market participants.
3. Limited Access in Rural AreasEastern Europe and rural regions often lack high-end sterilization facilities, slowing uniform adoption.
Competitive Landscape
The Europe medical device sterilization market includes:
-
Large multinational sterilization service providers
-
OEMs with in-house sterilization units
-
Specialized sterilization technology manufacturers
Key strategies include:
-
Capacity expansion
-
Adoption of advanced sterilization methods
-
Strategic collaborations with medical device companies
-
Strengthening quality management systems
-
Browse More Reports:
Global Connected Home Medical Sensor Device Market
Global Disseminated Intravascular Coagulation (DIC) Market
Global Dopamine Agonist Drug Market
Global Edible Insect Protein Ingredients Market
Global Fanconi Anaemia Treatment Market
Global Flake Graphite Market
Global GLP-1 Analogues Market
Global Histone Deacetylase Inhibitors Market
Global Hybrid Textile Market
Global Laser Capture Microdissection Market
Global Manual Resuscitators Market
Global Medical Device Outsourcing Market
Global Membrane Chromatography Market
Global Microbial Biosensors for Diagnostics Market
Global Mobile Biometrics Market
Future Outlook
The Europe medical device sterilization market is expected to continue expanding due to:
-
Increasing production of complex medical devices
-
Growth of minimally invasive and single-use devices
-
Higher investments in infection prevention technologies
-
Rapid adoption of automation and digital systems
As healthcare and manufacturing sectors evolve, sterilization will remain integral to ensuring patient safety and regulatory compliance, solidifying Europe’s position as a leader in advanced sterilization technologies.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com